Abstract
Primary mediastinal large B-cell lymphoma (PMBL) is an aggressive non-Hodgkin lymphoma which typically presents in young adults with a bulky mediastinal mass. Standard immunochemotherapy with R-CHOP21 alone is associated with a significant relapse risk if consolidative radiotherapy (RT) is omitted(Chan et al. Cancer Medicine. 2019; 8: 4626-4632). Dose-intensified regimens such as R-CHOP14, R-VACOP-B and R-DA-EPOCH have been reported to deliver better outcomes than R-CHOP21 with reduced requirement for RT and associated long-term risks, although direct randomised comparisons of regimens are lacking. Following publication of the prospective NCI trial which showed R-DA-EPOCH achieves excellent event-free (EFS) and overall survival (OS) without the need for RT (Dunleavy et al. NEJM. 2013; 368: 1408-1416), this regimen was adopted as a standard of care at multiple centres internationally. To date, few large real-world studies of R-DA-EPOCH with long-term follow-up have been published. Here we present a retrospective study of 122 adults from 9 centres in England, Scotland and Australia who were treated with first-line R-DA-EPOCH for PMBL 2010 - 2022 with a median follow-up of 3.2 (range: 0.3 - 11.6) years.
Patients (pts) had a median age of 34.1 years at diagnosis, with female preponderance (60%). Disease bulk (≥10cm) was present in 62%, 83% had raised LDH and 64% had stage 1-2 disease. Age-adjusted International Prognostic Index was 0,1,2 and 3 in 15%, 44%, 34% and 7% respectively.
The majority of pts received 6 cycles of R-DA-EPOCH (87%); only 4% completed <5 cycles. Of note, 13% of pts received R-CHOP as their first cycle of chemotherapy for stabilisation before commencing R-DA-EPOCH. The use of interim PET-CT (iPET) varied between institutions, with 72% (88/122) having an iPET after 2, 3 or 4 cycles (n=54, 30, 4, respectively). Likewise, end of treatment PET (ePET) was performed in 85% (104/122), being omitted in certain pts who had achieved a metabolic remission on iPET. Of the iPET pts, 41% achieved complete metabolic response (CMR) (Deauville Score [DS] ≤3), with no statistical difference in proportions between iPET2 and iPET3. 69% of iPET negative patients had an ePET and all remained DS ≤3 on ePET, while 59% (29/49) of iPET positive patients who had an ePET became DS≤3. Of all the ePET pts, 72% achieved DS ≤3. Consolidative RT was delivered more frequently in this real-world cohort than in the prospective trial, with 31% of pts receiving RT (16/75 (21%) of ePET DS≤3 pts and 16/29 (55%) of ePET DS4+ pts).
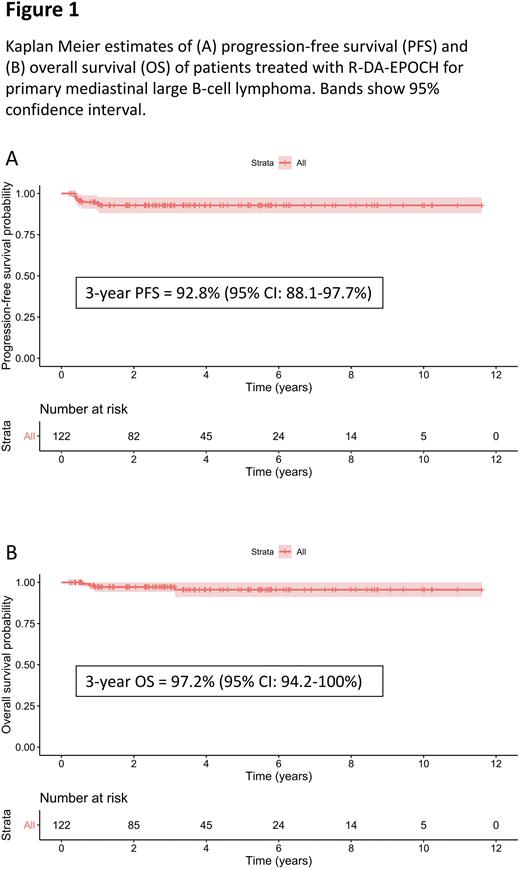
With a median follow-up of 3.2 years, 3-year PFS was 92.8% (95% CI: 88.1-97.7%), and 3-year OS was 97.2% (94.2-100%). There have been 8 progression events, with 6 cases of primary refractory disease at or before the ePET scan, and two relapses at 6 and 7 months after completing chemotherapy. Both of these pts had achieved ePET DS≤3 and had no RT. Of the 98 pts followed up beyond 1 year, no relapses have been observed. Of pts who had an initial cycle of R-CHOP, one has progressed with refractory disease after 5 x R-DA-EPOCH. 121/122 pts had at least 1 PET scan - 74% (90/121) achieved CMR on either iPET / ePET or both and the negative predictive values for relapse after achieving CMR on iPET and ePET was 97.2% and 97.3% respectively. Of 31 pts who failed to achieve a PET DS≤3 on either iPET (23 pts) or ePET (28 pts), 81% (25/31) have not relapsed. Of the 23 ePET DS4 patients, none have relapsed, but 14 / 23 had RT. Of the 8 ePET DS5 patients, 3 remain disease free with 2/3 receiving RT. Of the 10 ePET DS4-5 pts who did not receive RT, 70% (7/10) achieved CMR at mean 4.4 months after final chemotherapy, whilst 30% (3/10) pts remain PET positive but have not required further treatment.
There have been 4 deaths in total (3 refractory disease and 1 death due to complications of allogeneic transplant). Two second malignancies (Hodgkin lymphoma at 4 years, serous ovarian malignancy at 3.5 years) have been reported and pregnancy data will be presented.
In conclusion, with this large multi-centre real-world dataset with long-term follow-up, we demonstrate that R-DA-EPOCH is a highly effective regimen to treat PMBL and that potentially, ePET has little additional utility in pts with CMR on iPET. The use of RT appears more widespread in real-world practice as the majority of pts who were ePET DS4+ were treated with RT. Pts who achieved a metabolic remission on either interim or end of treatment PET/CT had a very low relapse risk and no relapses beyond 1 year were reported.
Disclosures
Santarsieri:Takeda: Other: Conference funding 2021; Janssen: Honoraria. Lewis:AstraZeneca: Consultancy, Honoraria; IQVIA: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Patents & Royalties; Loxo-Lilly: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Novartis: Patents & Royalties; Roche: Consultancy, Honoraria. Nagumantry:Alexion: Honoraria; Janssen: Honoraria. Shah:Janssen: Consultancy; Abbvie: Consultancy. Cheah:TG Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. McKay:Abbvie: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Celgene/BMS: Consultancy; Epizyme: Consultancy; Gilead/Kite: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Recordati Rare Diseases: Consultancy; Roche: Consultancy; Takeda: Consultancy. Dickinson:Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD: Consultancy; Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD, Amgen: Honoraria; Roche, Novartis, Kite, Gilead, MSD, Takeda, Celgene: Research Funding. Seymour:AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genor Biopharma: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Follows:Janssen: Honoraria; Abbvie: Honoraria; Roche: Honoraria; Astra Zeneca: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal